 1) vascular
1) vascular2) nonvascular
plants that lack tube-like cells and do not have “true” roots, stems, & leaves
2 Main Types of Nonvascular Plants
A small simple nonvascular plant that has both stems and leaves…but NO ROOTS
Mosses are considered nonvascular and are grouped with liverworts because:
a) their vascular (inner) tissue is very simple
b) they both have similar life cycles
Nonvascular Plants: Moss
fine soft stems
leaves are only one or two cells thick
leaves grow from all sides of the stem
moss species can be classified by their leaves based on:
a) Placement of leaves on the stem
b) Shape of leaves
Nonvascular Plants: Mosses
4 uses of mosses1) Food for animals
EX: worms & snails eat moss
2) They help hold soil in place to keep it from washing away
moss that is added to soil to increase the amount of water it holds
Nonvascular Plants: Mosses
Moss Life CycleSexual reproduction-
form a new organism by the union of 2 reproductive cells
1) female reproductive cell
2) male reproductive cell
Both are located at the tips of the mosses leafy stem
fertilization
the joining of the egg and the sperm
Moss Life Cycle
sperm and egg of mosses form at the tips of the leafy stemssperm of mosses and liverworts must swim to the egg for fertilization
fertilization
2) A stalk grows from a fertalized egg
3) Brown capsule forms at the end of the stalk (stem)
4) Brown capsule contains spores
5) Spores are blown away from parent plant…by wind
6) They land and grow into new leafy plants
2 Main Types of Nonvascular Plants
2) liverwort-A small simple nonvascular plant that do NOT have roots, stems, or leaves
H2O and other materials are distributed throughout their bodies by:
a) osmosis
b) diffusion
Nonvascular Plants: liverworts
General Characteristics facts about liverworts1) simplest nonvascular plant
2) flat body
3) slippery layer of green cells on the ground
4) leaves grow in 2-3 flattened rows along the stem
What two types of nonvascular plants are shown on this rock?
1) Moss
2) Liverwort
Slide Questions Part Two (slides 27-36)
Directions: Answer the following questions.1) List the main types of plants
2) Define nonvascular.
3) Define moss and list 3 of the 5 main characteristics of mosses.
4) Why are mosses considered nonvascular if they appear to have a stem and a leaf?
5) List the two ways that moss species are classified.
Slide Questions Part Two (slides 27-36)
Directions: Answer the following questions.6) List the 4 uses of moss.
7) Explain how mosses reproduce.
8) Define fertilization.
9) Define liverwort. How so they take in water?
10) List two of the four characteristics of liverworts.
Acids & Bases
By Robert McGeeOur Goals for today
To determine the difference between Acids & BasesDiscuss the importance of studying Acids & Bases
Perform an experiment dealing with Acids & Bases
What is the pH scale?
The pH scale measures how acidic or basic a solution is.The pH scale
The pH scale is the concentration of hydrogen ions in a given substance.Identifying Acids and Bases
Acids have a ph from 0-7Lower pH value indicates a stronger acid.
Bases have a pH from 7-14
Higher pH value indicates a stronger base.
Definitions of Acids and Bases
An acid is a substance that breaks into ions in an aqueous solution.A Base (alkaline) is a substance that breaks into ions in an aqueous solution.
Note: aqueous solution is any solution where is the solvent.
Did we Miss something??
What happens when the pH of a substance is 7?Ans: A pH level of 7 indicates a Neutral Substance i.e: Water!
Test Your Knowledge
What is the range of an ACID on the pH scale?What is the range of a BASE and what is another name for a BASE?
Ans: 7-14, Alkaline
Characteristics Of Acids
A sour taste.Acids can be characterized by:
It turns blue litmus paper red
It tastes sour. Try drinking lemon juice (citric acid)
Characteristics of Bases
A Base is characterized by:A bitter taste. (Milk of Magnesia)
It feels slippery. (Soapy Water)
It turns Red Litmus Blue.
Why Learn about Acids & Bases?
What do you think is the pH level of tap water?The pH of a swimming pool must be checked periodically. Why?
Is it important for Lakes & Rivers to maintain a certain pH?
Today’s Experiment
Test the pH of Pepsi, tap water, and drain cleanerGOOD LUCK!!!
SNEAK PREVIE
ANCIENT CIVILIZATIONS
Ancient MesopotamiaAncient Egypt
Ancient India
Ancient China
Ancient Greece
Ancient Rome
ANCIENT MESOPOTAMIA
Oldest known civilizationBabylon
Gilgamesh
Hammarabi
Nebuchadnezzar
Ziggurat
Hanging gardens
Models of the Atom a Historical Perspective
Early Greek Theories
400 B.C. - Democritus thought matter could not be divided indefinitely.This led to the idea of atoms in a void.
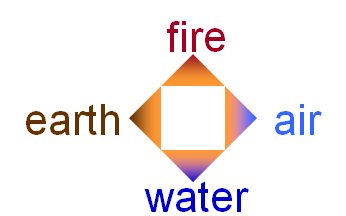
350 B.C - Aristotle modified an earlier theory that matter was made of four “elements”: earth, fire, water, air.
Aristotle was wrong. However, his theory persisted for 2000 years.
John Dalton
1800 -Dalton proposed a modern atomic modelbased on experimentation not on pure reason.
All matter is made of atoms.
Atoms of an element are identical.
Each element has different atoms.
Atoms of different elements combine in constant ratios to form compounds.
Atoms are rearranged in reactions.
His ideas account for the law of conservation of mass (atoms are neither created nor destroyed) and the law of constant composition (elements combine in fixed ratios).
Adding Electrons to the Model
Materials, when rubbed, can develop a charge difference. This electricity is called “cathode rays” when passed through an evacuated tube (demos).These rays have a small mass and are negative.
Thompson noted that these negative subatomic particles were a fundamental part of all atoms.
Dalton’s “Billiard ball” model (1800-1900)
Atoms are solid and indivisible.
Thompson “Plum pudding” model (1900)
Negative electrons in a positive frameworkThe Rutherford model (around 1910)
Atoms are mostly empty space.
Negative electrons orbit a positive nucleus
Bohr’s model
Electrons orbit the nucleus in “shells”Electrons can be bumped up to a higher shell if hit by an electron or a photon of light.
There are 2 types of spectra: continuous spectra & line spectra. It’s when electrons fall back down that they release a photon. These jumps down from “shell” to “shell” account for the line spectra seen in gas discharge tubes (through spectroscopes).
Atomic numbers, Mass numbers
There are 3 types of subatomic particles. We already know about electrons (e–) & protons (p+). Neutrons (n0) were also shown to exist (1930s).They have: no charge, a mass similar to protons
Elements are often symbolized with their mass number and atomic number
These values are given on the periodic table.
For now, round the mass # to a whole number.
These numbers tell you a lot about atoms.
# of protons = # of electrons = atomic number
# of neutrons = mass number – atomic number
Isotopes and Radioisotopes
Atoms of the same element that have different numbers of neutrons are called isotopes.Due to isotopes, mass #s are not round #s.
Li (6.9) is made up of both 6Li and 7Li.
Often, at least one isotope is unstable.These types of isotopes are called radioisotopes
Q- Sometimes an isotope is written without its atomic number - e.g. 35S (or S-35). Why?
Q- Draw B-R diagrams for the two Li isotopes.




















Post a Comment